By Dorothy Musyoka
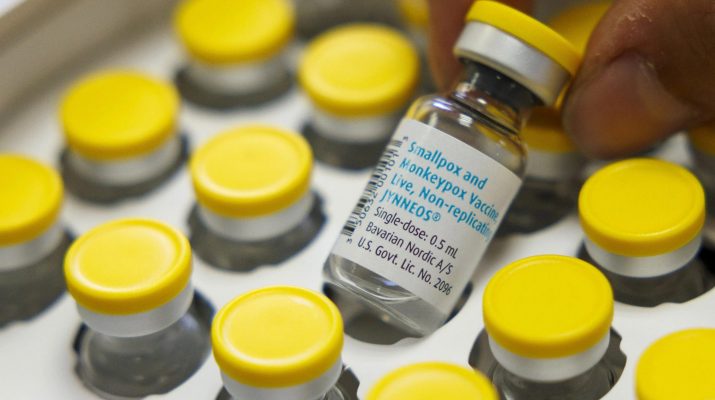
The World Health Organization (WHO) has announced the prequalification of the MVA-BN vaccine, making it the first vaccine against mpox to be added to the WHO’s prequalification list.
This crucial step aims to increase access to the vaccine in communities with urgent need, particularly in Africa, where ongoing outbreaks continue to pose significant public health risks.
The WHO Director-General, Dr. Tedros Adhanom Ghebreyesus, emphasized the importance of scaling up vaccine procurement, donations, and rollout to ensure equitable access.
“This first prequalification of a vaccine against mpox is an important step in our fight against the disease, both in the context of the current outbreaks in Africa, and in future,” said Dr Tedros Adhanom Ghebreyesus.
The prequalification approval, based on assessments by the European Medicines Agency and information submitted by the manufacturer, Bavarian Nordic A/S, is set to accelerate the availability of the vaccine.
“We now need urgent scale up in procurement, donations and rollout to ensure equitable access to vaccines where they are needed most, alongside other public health tools, to prevent infections, stop transmission and save lives,” added WHO Director General.
The MVA-BN vaccine, suitable for adults aged 18 and over, is administered in a two-dose schedule four weeks apart.
“The MVA-BN vaccine can be administered in people over 18-years of age as a 2-dose injection given 4 weeks apart. After prior cold storage, the vaccine can be kept at 2–8°C for up to 8 weeks,” noted WHO.
Addittionally the vaccine is recommended for use in outbreak settings for persons at high risk of exposure, including off-label use in younger age groups, pregnant women, and immunocompromised individuals where benefits outweigh risks.
According to WHO, 103,000 mpox cases have been reported globally since the onset of the outbreak in 2022, including significant numbers in Africa, WHO’s prequalification of the MVA-BN vaccine represents a critical development in the fight against the disease.